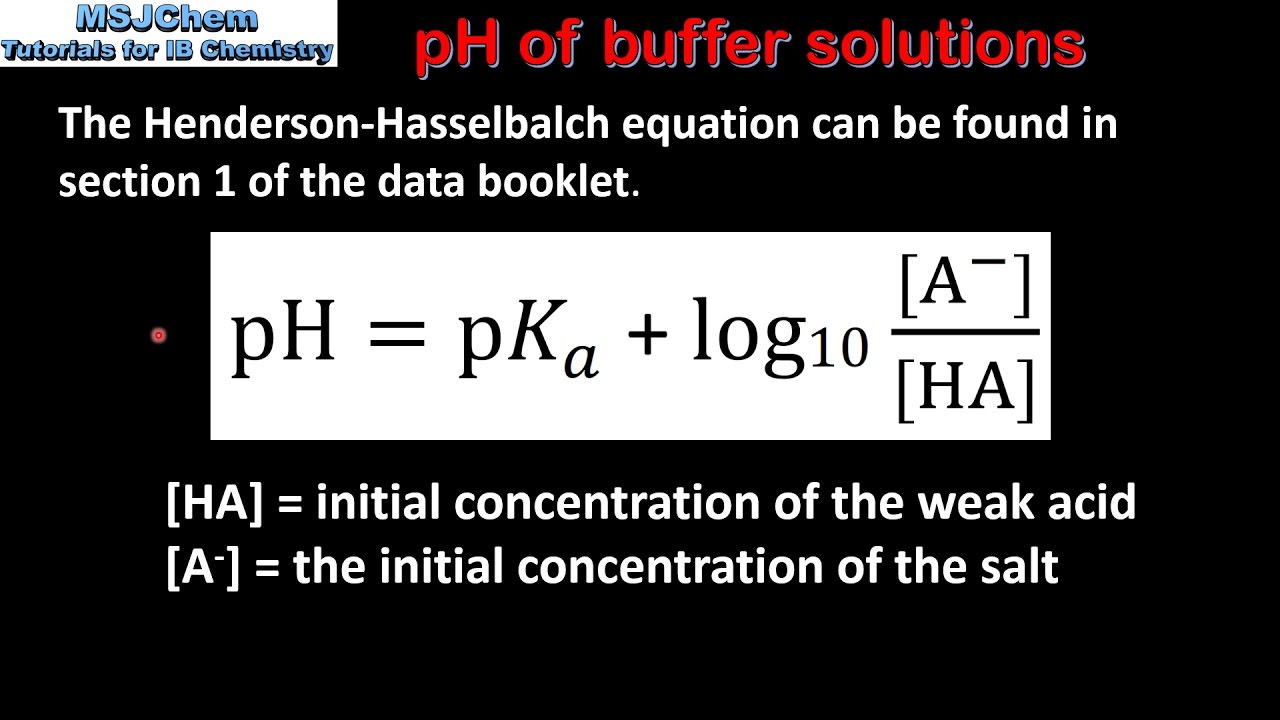
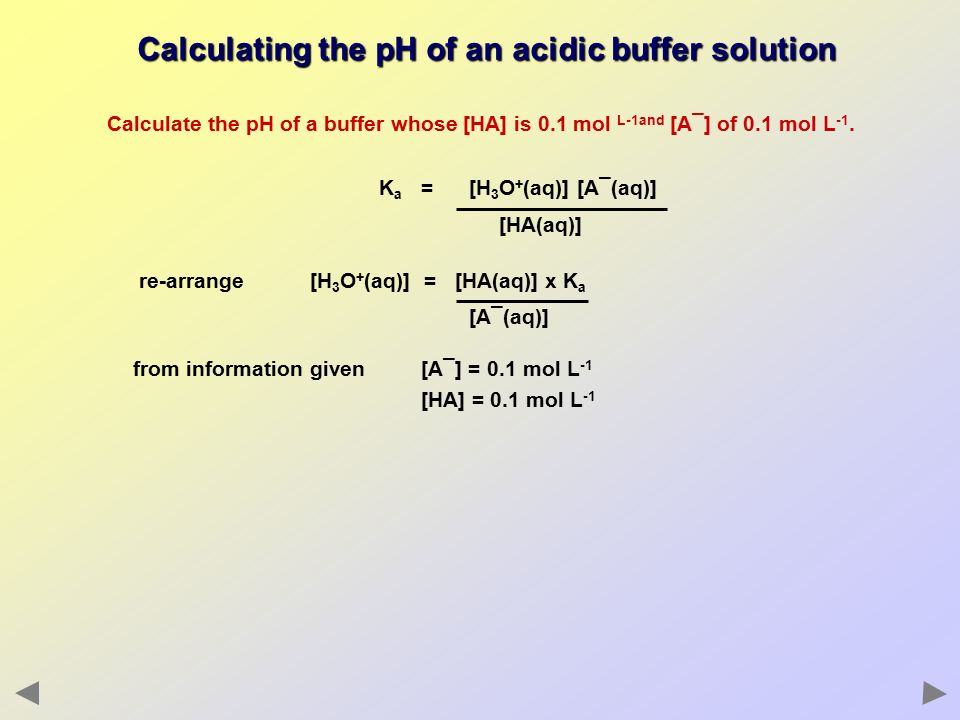
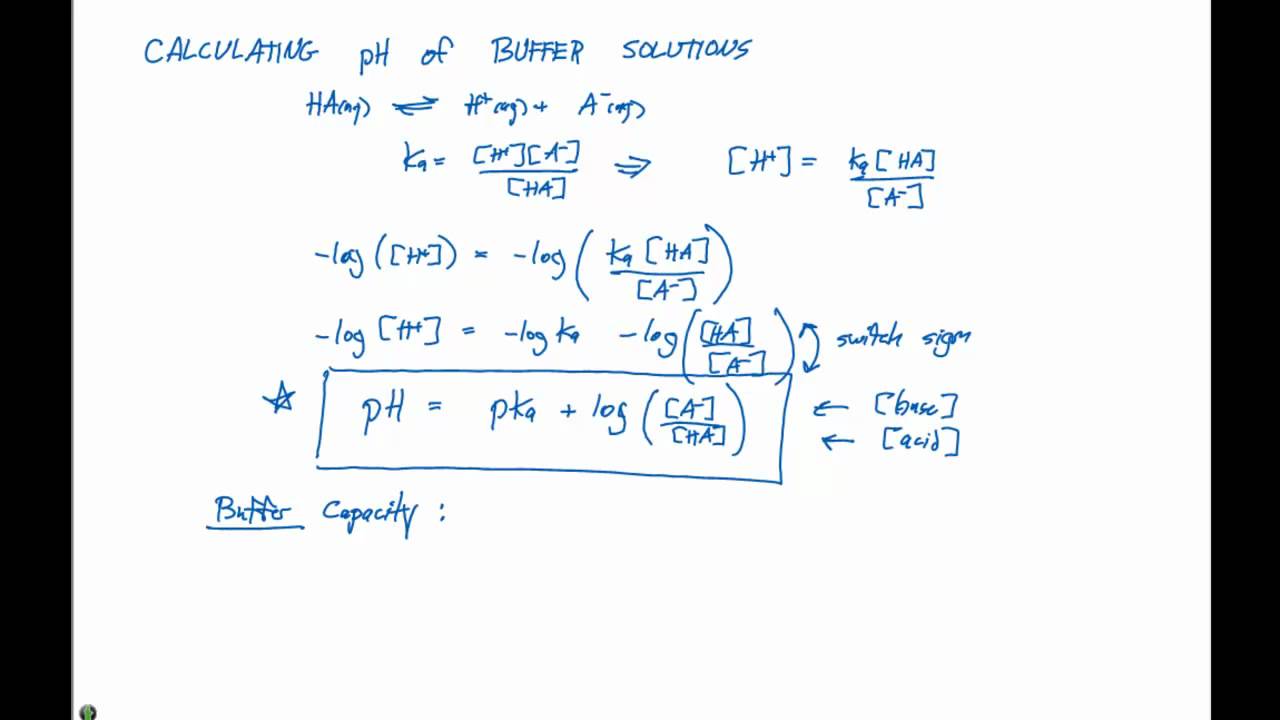
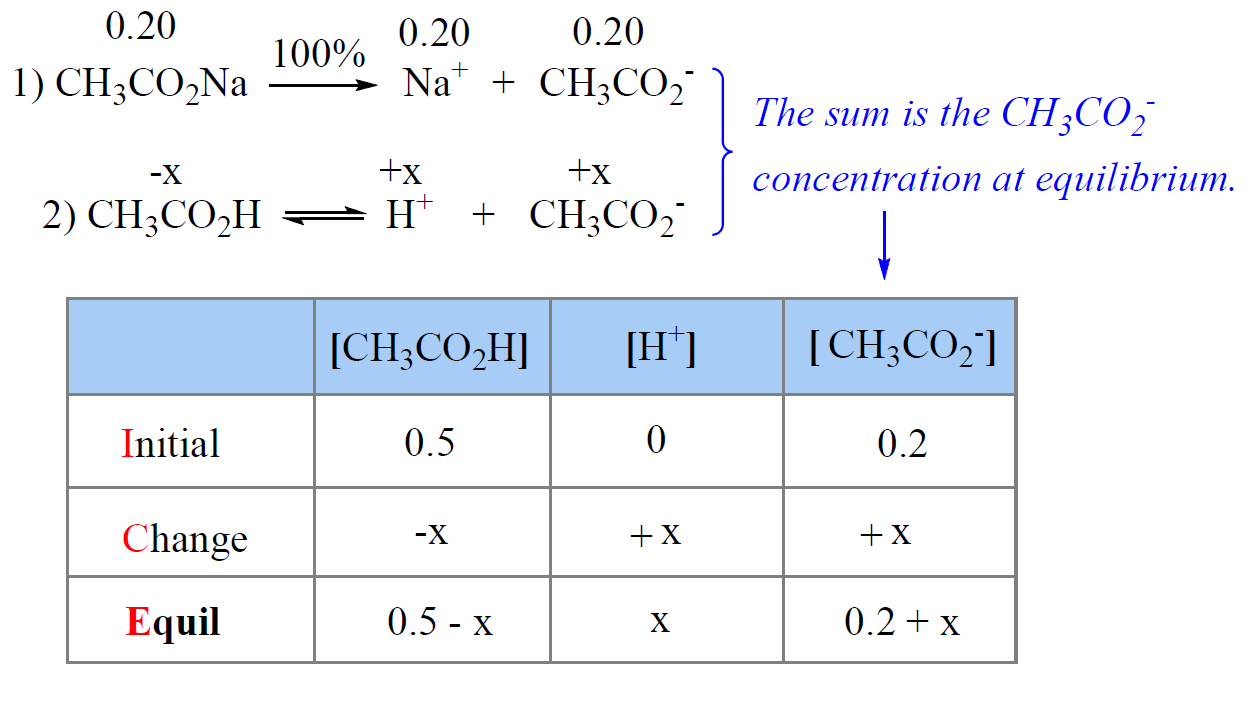
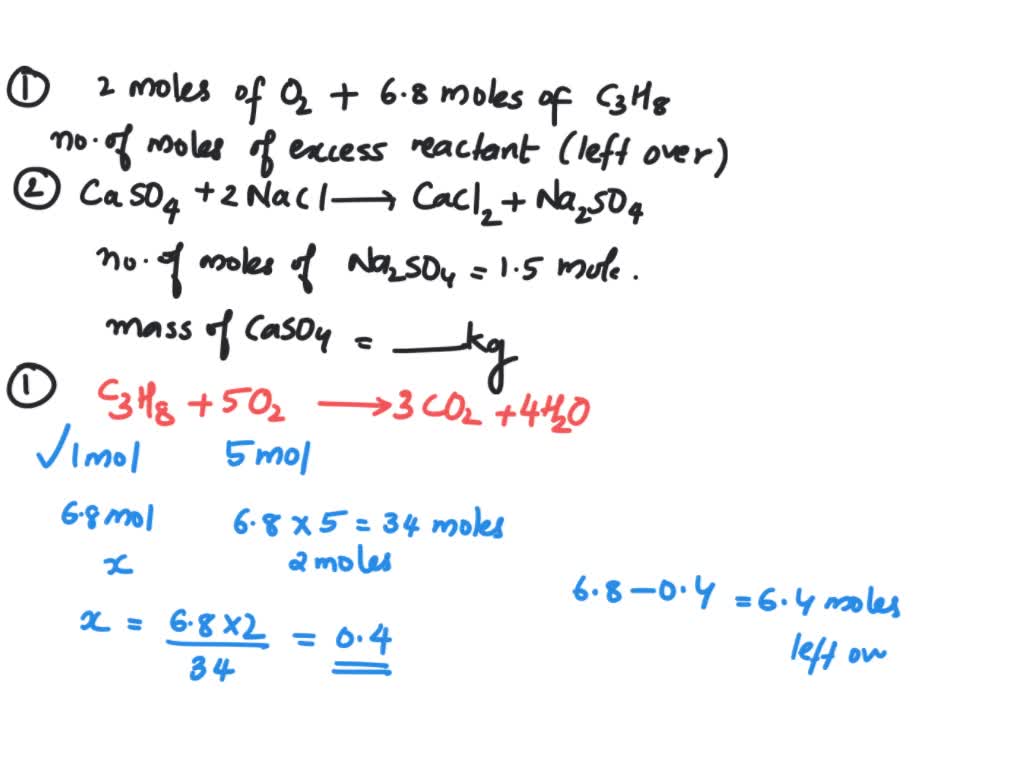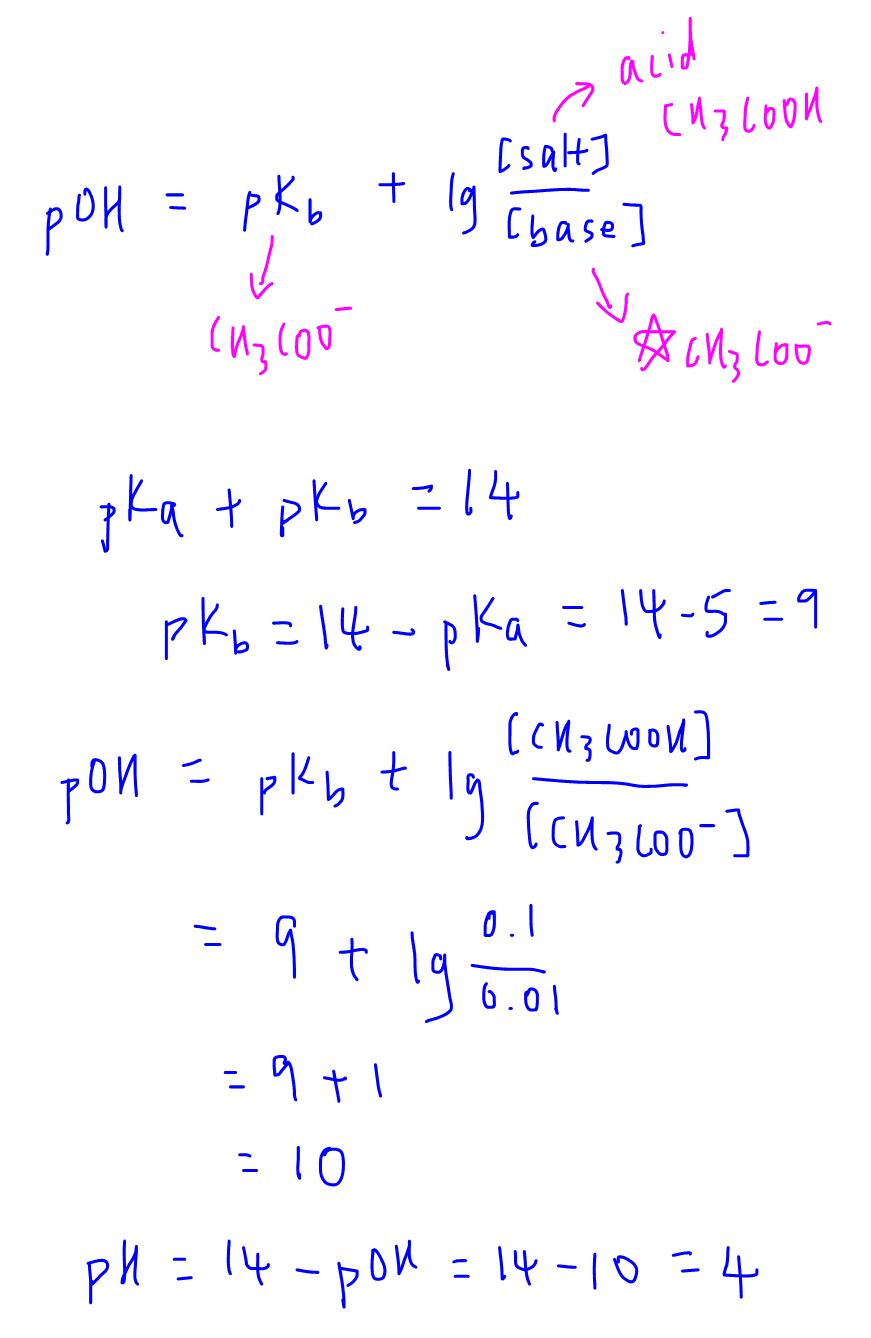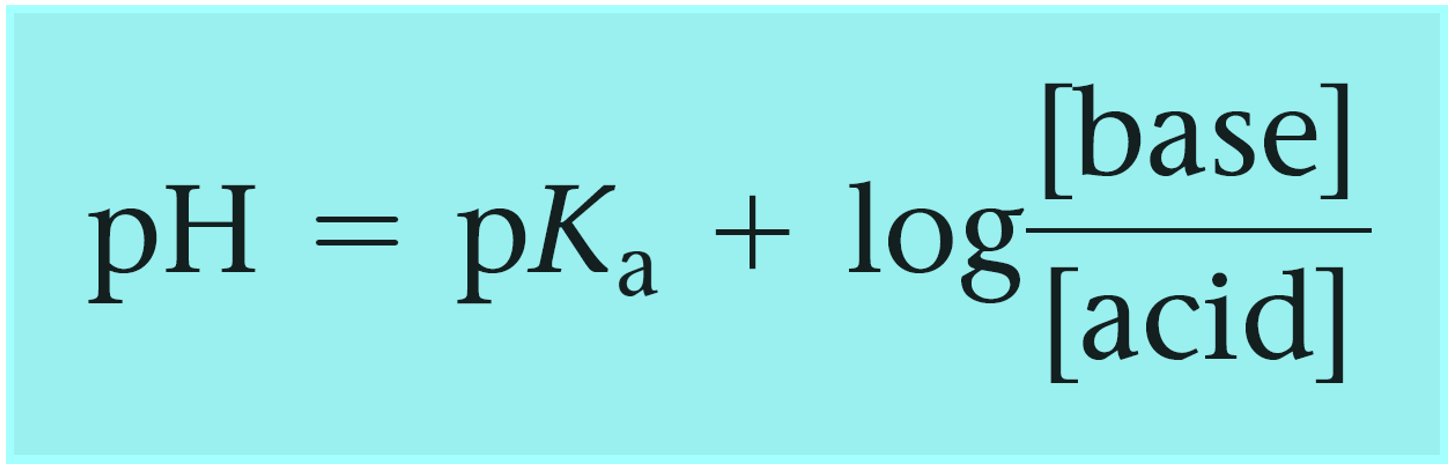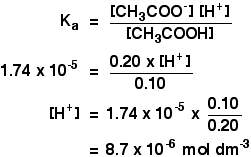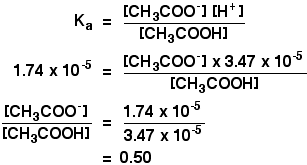A buffer solution was prepared by dissoving 0.02 mol propionic acid and 0.015 mol sodium propionate in enough water to make 1.00L of solution. (Ka for propoinic acid is 1.34 × 10^-5 ).
![Calculate the pH of a buffer solution prepared by dissolving 30g of Na2CO3 in 500 mL of an aqueous solution containing 150 mL of 1M HCl . Ka for: HCO3 = 5.63 × 10^-11 [ log 133150 = - 0.05 ] Calculate the pH of a buffer solution prepared by dissolving 30g of Na2CO3 in 500 mL of an aqueous solution containing 150 mL of 1M HCl . Ka for: HCO3 = 5.63 × 10^-11 [ log 133150 = - 0.05 ]](https://dwes9vv9u0550.cloudfront.net/images/6404140/adc2d8b1-954e-4b3c-b9c4-733c3311ce8f.jpg)
Calculate the pH of a buffer solution prepared by dissolving 30g of Na2CO3 in 500 mL of an aqueous solution containing 150 mL of 1M HCl . Ka for: HCO3 = 5.63 × 10^-11 [ log 133150 = - 0.05 ]