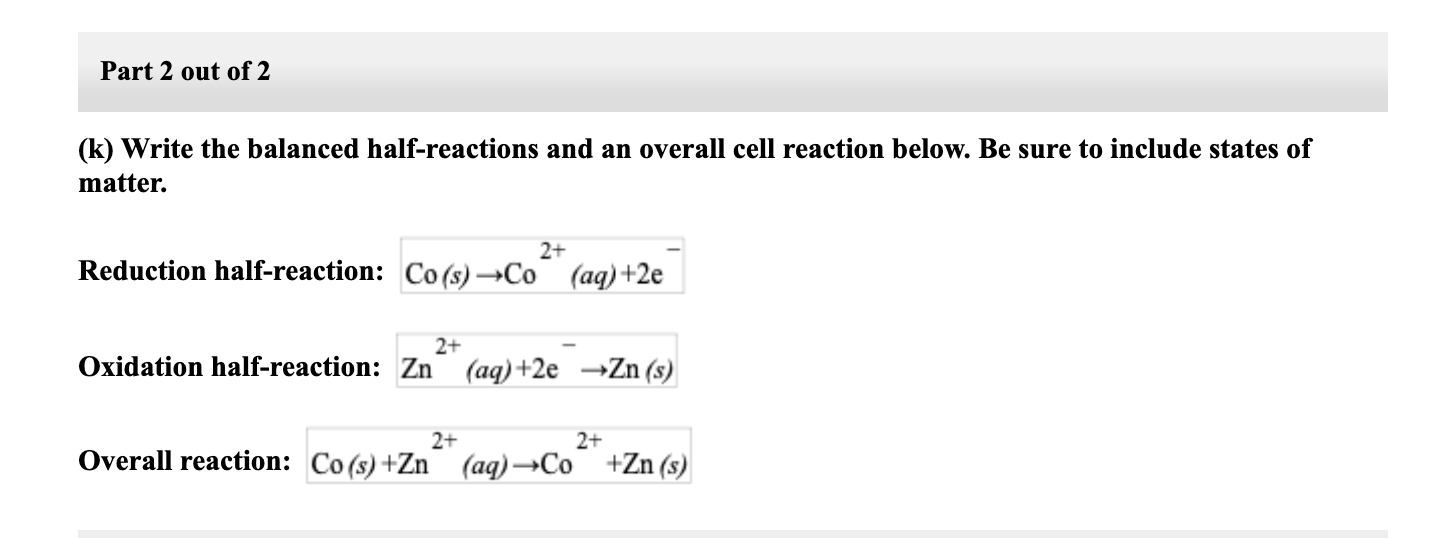
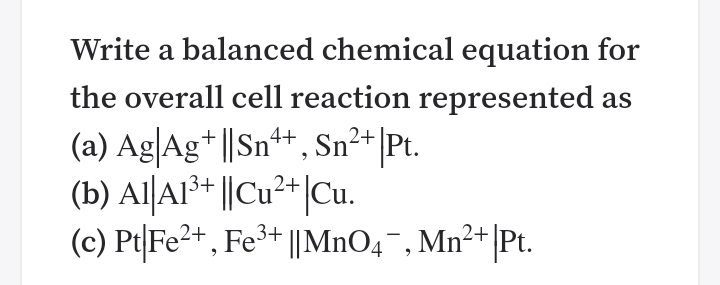Write electrode reactions and overall cell reaction for Daniel cell you learnt in standard XI. - Sarthaks eConnect | Largest Online Education Community
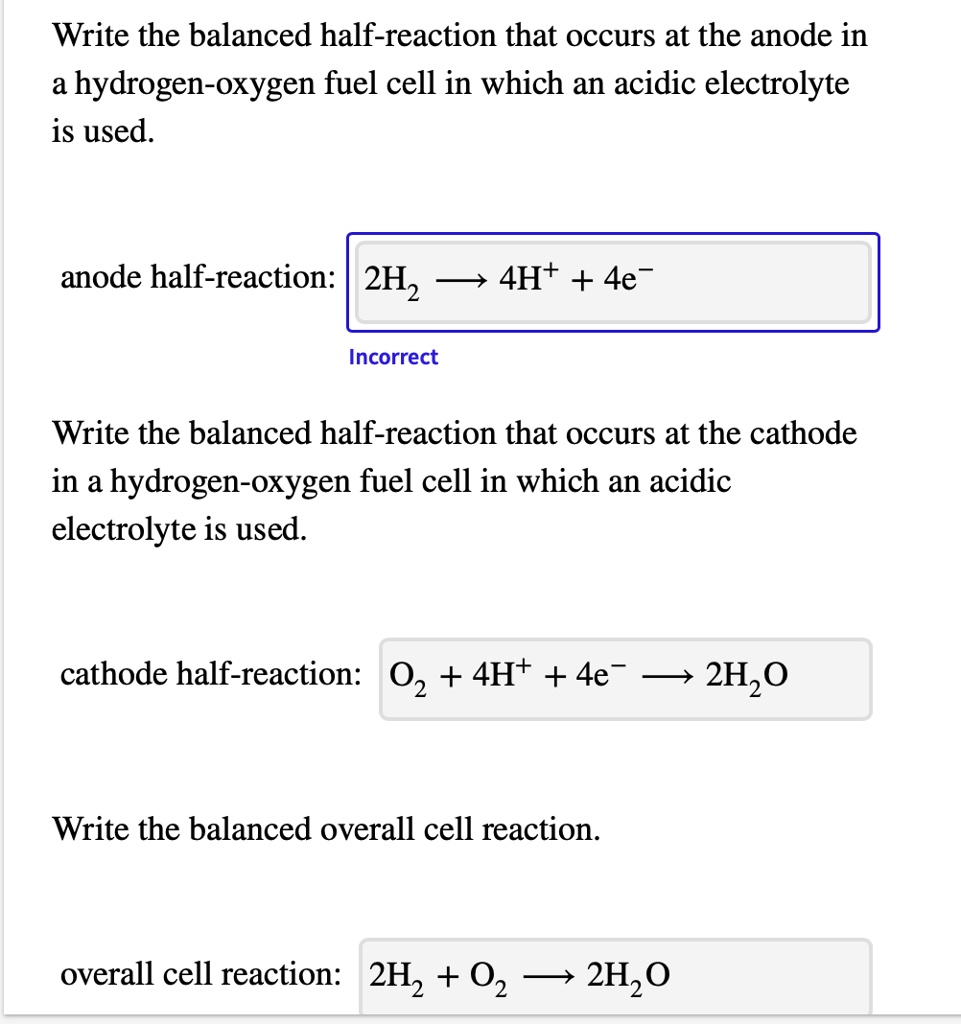
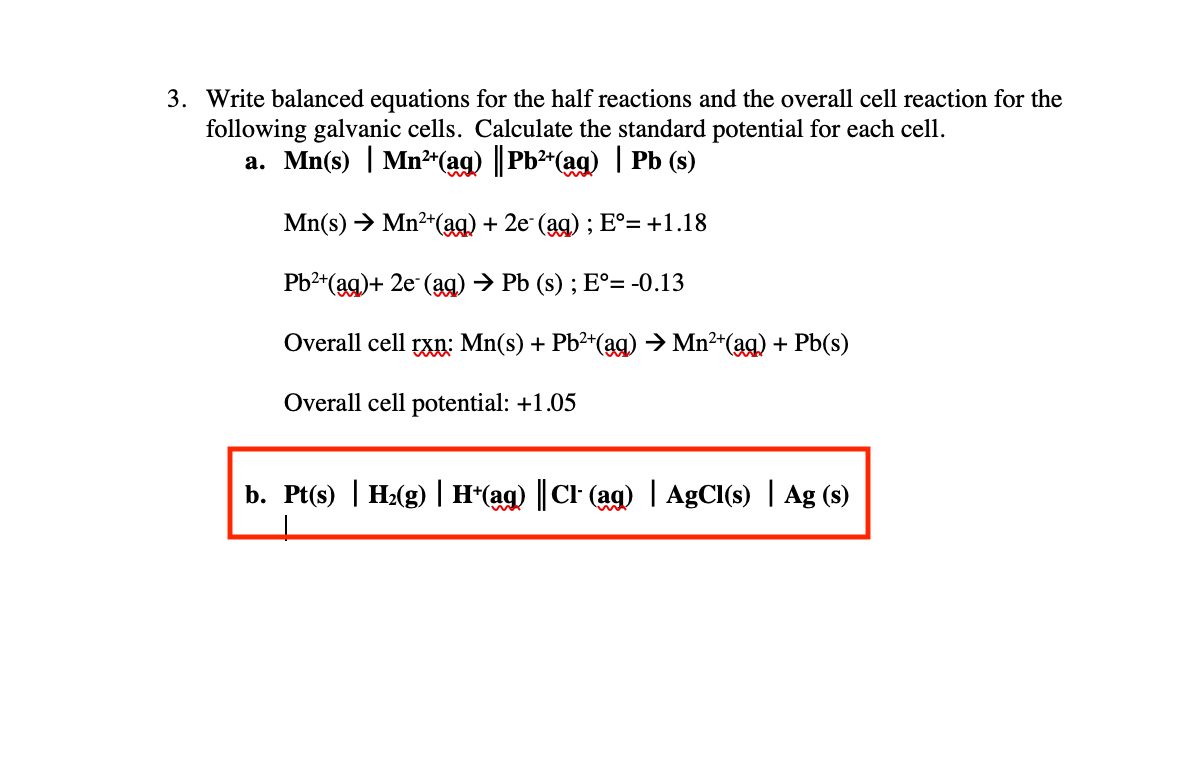
SOLVED: Write the balanced half-reaction that occurs at the anode in a hydrogen-oxygen fuel cell in which an acidic electrolyte is used. anode half-reaction: 2H2 + 4H+ + 4e- Write the balanced

write the half cell reaction and the overall cell reaction for the electrochemical cell : - Brainly.in

Write Electrode Reaction and Net Cell Reaction for Fuel Cell. Calculate E.M.F. of the Following Cell at 25 °C - Chemistry | Shaalaa.com

OneClass: o Write the overall cell reaction for the galvanic cell given below Pt(s) I H2(9) I H*(oq) ...
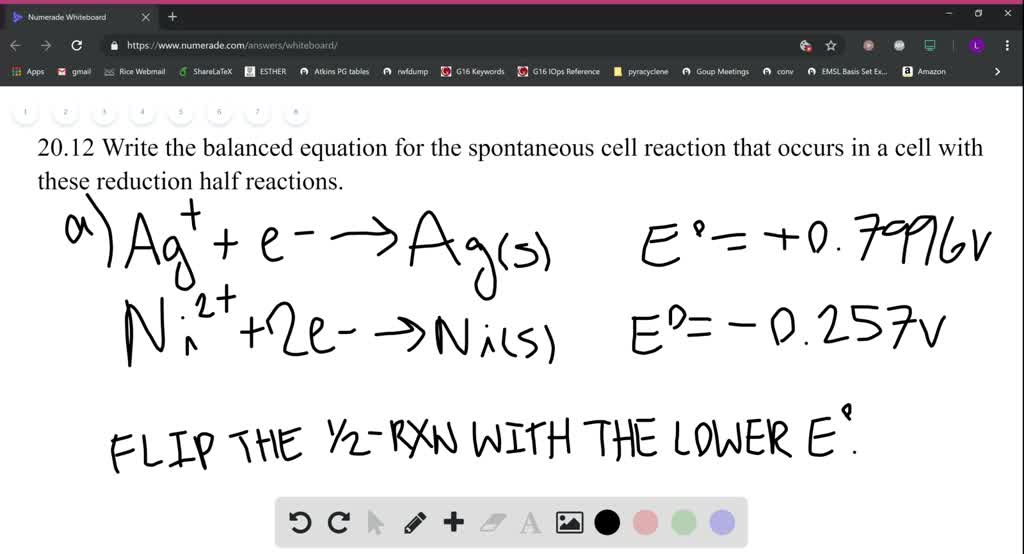
SOLVED:Write the balanced equation for the spontaneous cell reaction that occurs in a cell with these reduction half-reactions. a. A g^+(a q)+e^- →A g(s) and N i^2+(a q)+2 e^- →Ni(s) b. Mg^2+(aq)+2

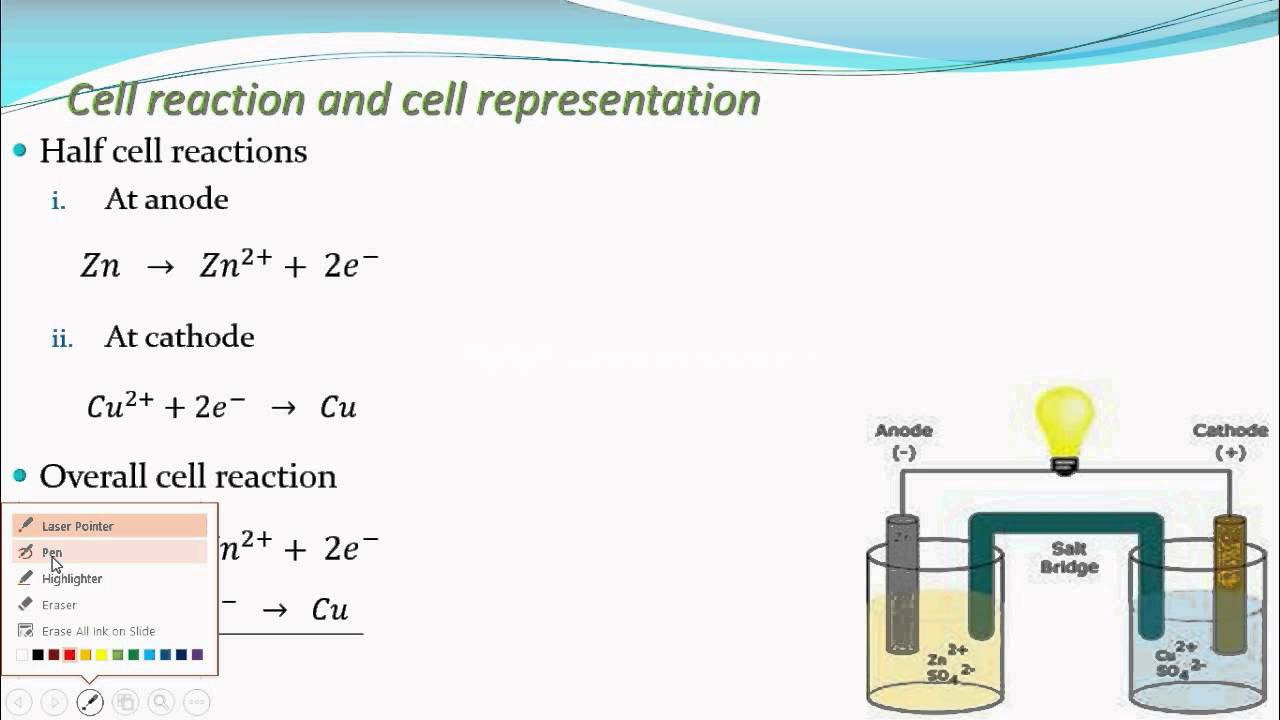
How to Write Overall cell reaction/Net cell reaction and Half-cell reactions of a Galvanic cell? - YouTube
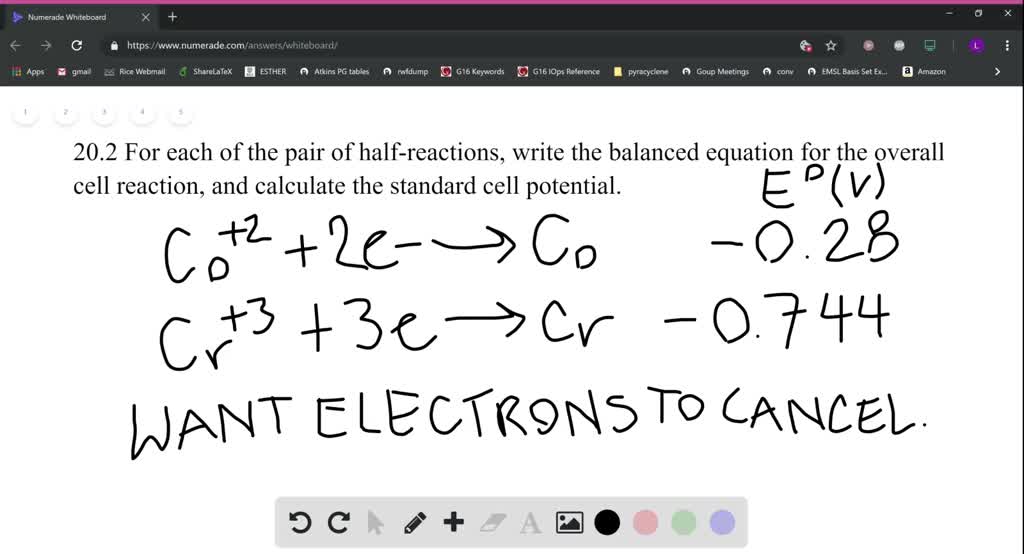
SOLVED:For each of these pairs of half-reactions, write the balanced equation for the overall cell reaction, and calculate the standard cell potential. Describe the reaction using cell notation. Refer to Chapter 19

SOLVED: 17. (7 points) Given the following two half-reactions: Pb2-(aq) + 2e- -> Pb(s) (0.126 V) and Fe3+(aq) + e- -> Fe2+(s) (E' = +0.771 V), write the overall reaction in the

Write the cell reaction and calculate the emf of the cell Pt |H2 (g, 1 atm) | H^ + (0.5 M) || KCl (1 M) |Hg2Cl2 (s) | Hg (l) | Pt at 25^o C, E = 0.28 V

SOLVED:Write a balanced net ionic equation for the overall cell reaction represented by (a) Cd|Cd^2+ Sb^3+| Sb (b) Pt|Cu^+, Cu^2+ Mg^2+| Mg (c) Pt|Cr^3+, Cr2 O7^2- ClO3^-, Cl^-| Pt (acid)