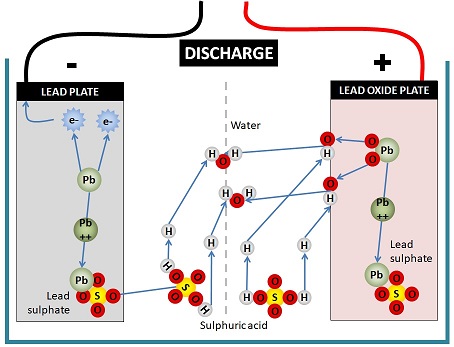
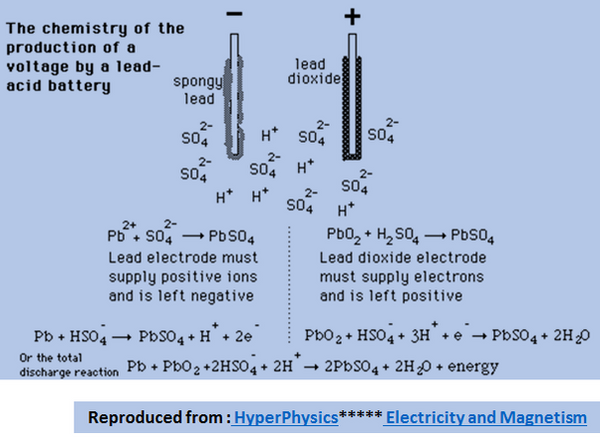
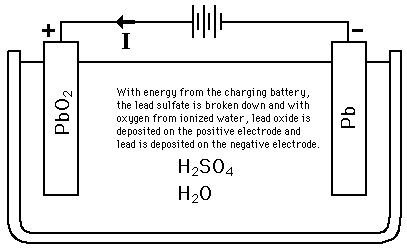
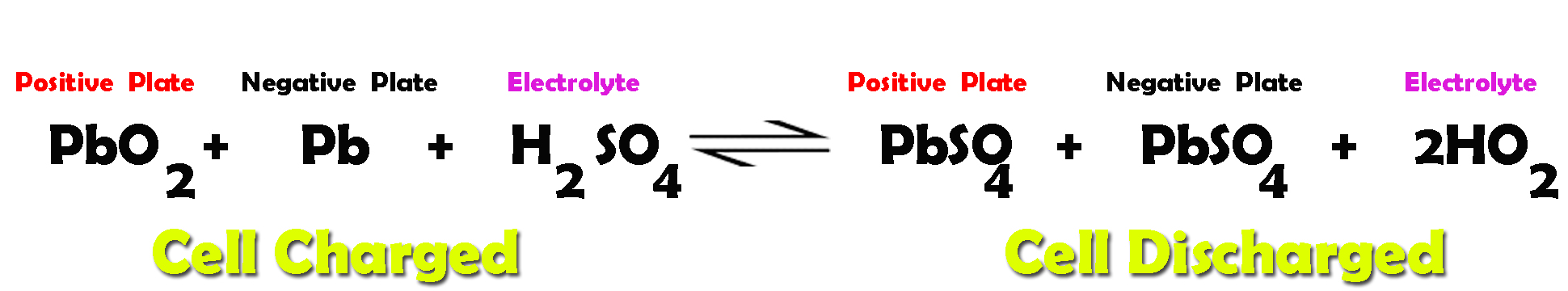
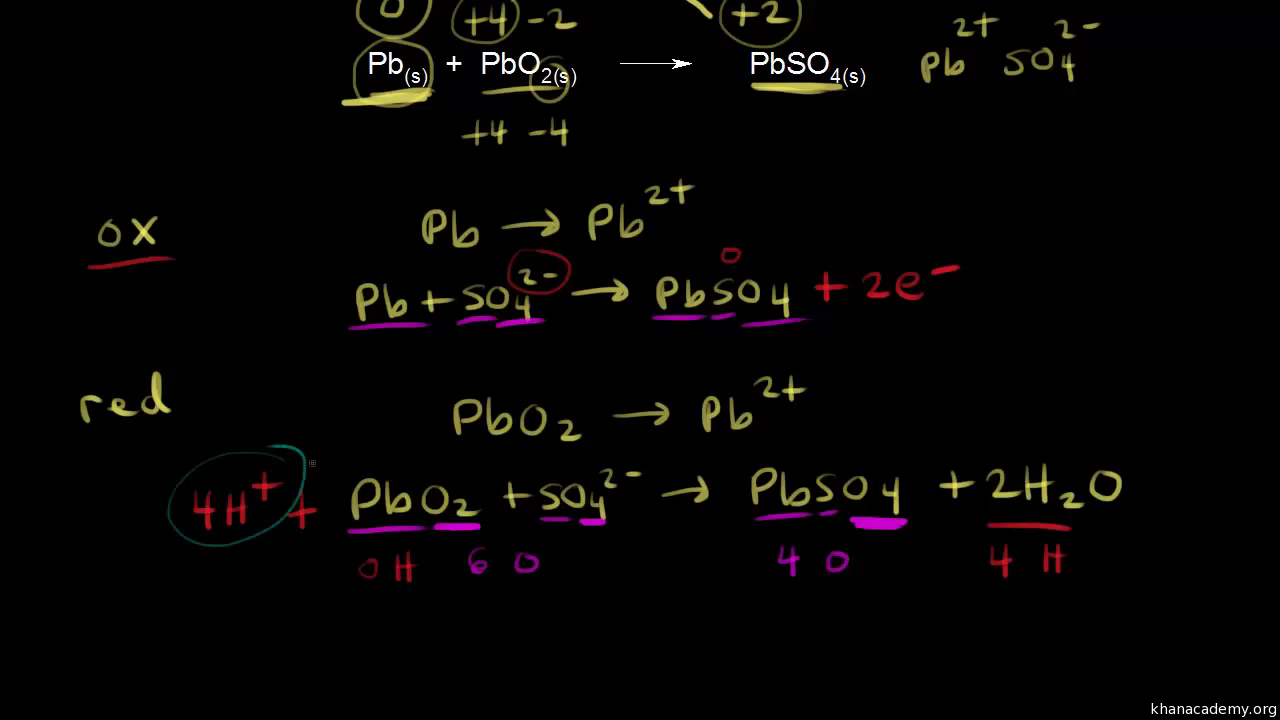
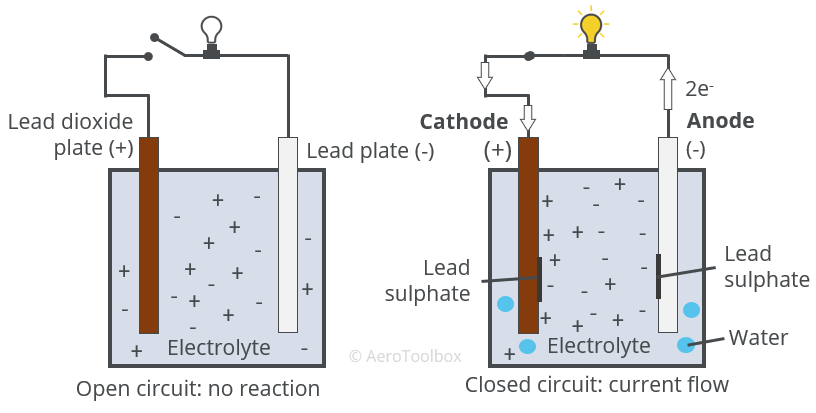
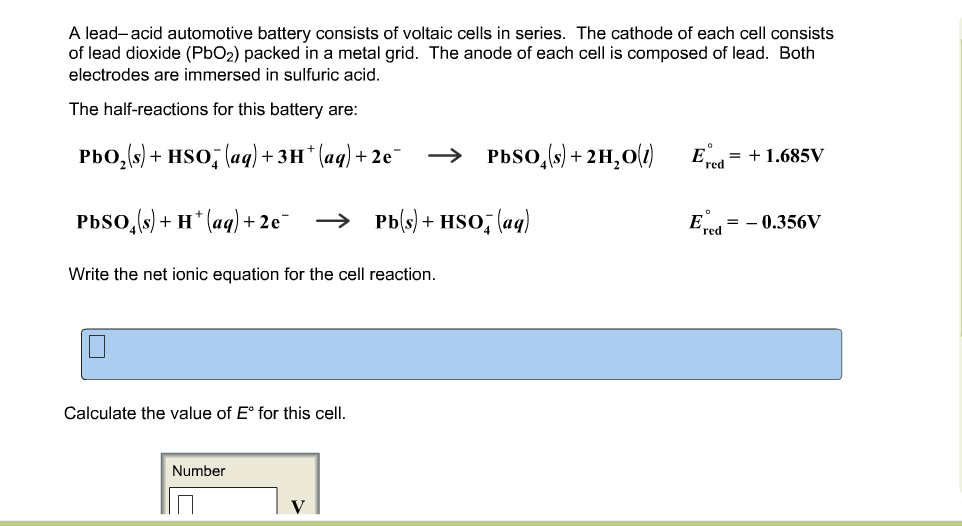
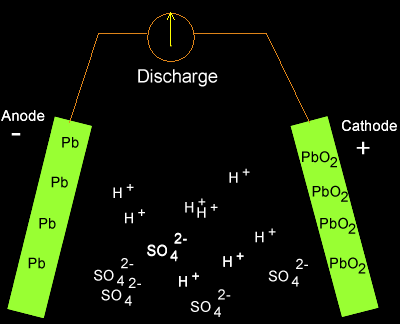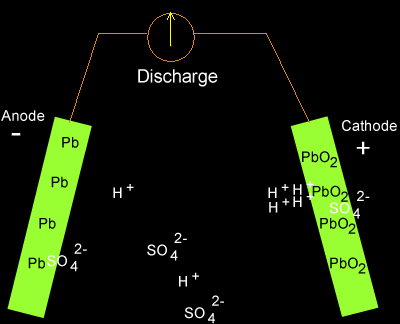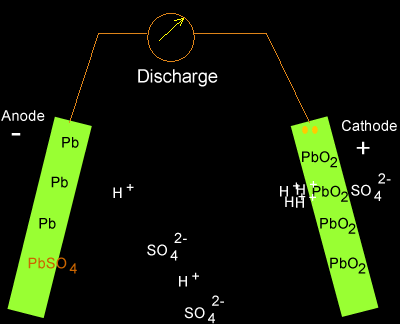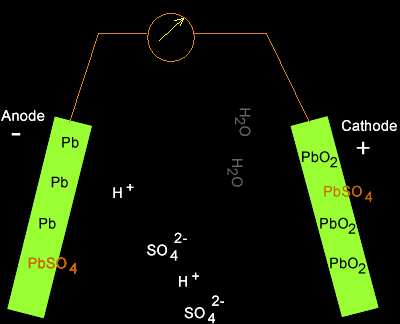
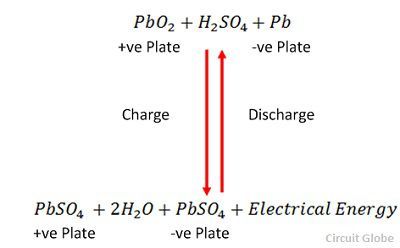
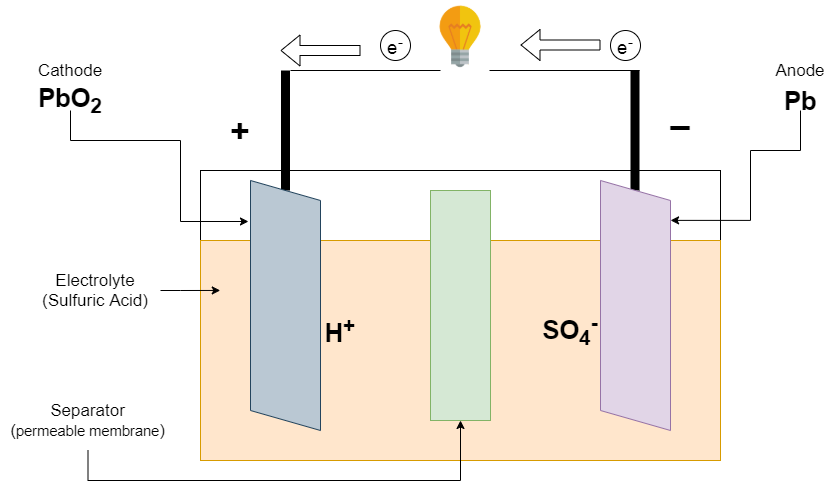
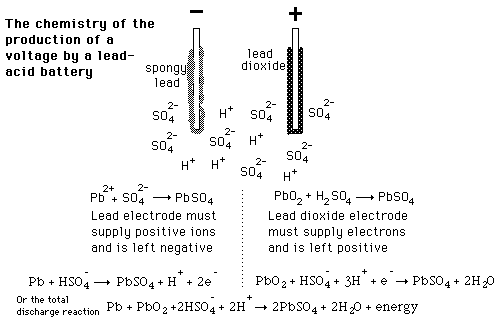![SOLVED:The overall reaction in the lead storage battery is Pb(s)+PbO2(s)+2 H^+(a q)+2 HSO4^-(a q) ⟶2 PbSO4(s)+2 H2 O(l) Calculate ℰ at 25^∘ C for this battery when [H2 SO4]=4.5 M, that is, [ SOLVED:The overall reaction in the lead storage battery is Pb(s)+PbO2(s)+2 H^+(a q)+2 HSO4^-(a q) ⟶2 PbSO4(s)+2 H2 O(l) Calculate ℰ at 25^∘ C for this battery when [H2 SO4]=4.5 M, that is, [](https://cdn.numerade.com/previews/ef396843-733f-4d23-8732-73bb08581931.gif)
SOLVED:The overall reaction in the lead storage battery is Pb(s)+PbO2(s)+2 H^+(a q)+2 HSO4^-(a q) ⟶2 PbSO4(s)+2 H2 O(l) Calculate ℰ at 25^∘ C for this battery when [H2 SO4]=4.5 M, that is, [

Question Video: Identifying the Overall Equation for the Reaction That Occurs When Lead–Acid Batteries Discharge | Nagwa